| front |1 |2 |3 |4 |5 |6 |7 |8 |9 |10 |11 |12 |13 |14 |15 |16 |17 |18 |19 |20 |21 |22 |23 |24 |review |
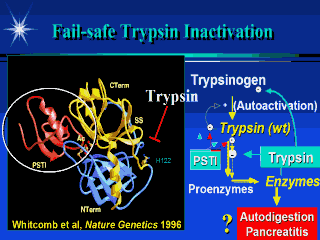 |
The trypsin molecule is illustrated in
yellow and blue and is shaped like a pac-man with the jaws opening to the left. If a
protein chain with an arginine or lysine inters the jaw it is rapidly cut into two pieces.
Tryspinogen is made by the pancreas in an inactive form. It is one of the most important enzymes because one it is activated to trypsin in the intestine, it is responsible for activating nearly all of the other digestive enzymes to their active form, in the intestine. If trypsinogen becomes active in the pancreas it could activate all of the other digestive enzymes and cause the pancreas to digest itself. Forturnately, the pancreas also makes a trypsin inhibitor (PSTI - in red) that plugs the active site and prevents trypsin from activating other trypainogen molecules and all the other enzymes. However, the amount of PSTI is limited, so another protective mechanism is necessary. If too much trypsin is active, trypsin begins attacking itself at arginine #117 (R117). Cutting the connecting chain splits the trypsinogen molecule in half, permanently inactivating it, and saving the pancreas from auto-digestion. Solving the genetic code for hereditary pancreatits proved that the mutation was at R117 changing it to Histidine 117. |