“Necessity is the mother of invention” is a popular English proverb that we realize is true when crises occur or when we go through difficulties in our lives. In recent times, the increase in global fuel prices is a recurring crisis, in addition to persistent climate change due to carbon dioxide emissions, which led to the Paris Climate Agreement 2015. The main aim of the agreement is to enhance the global response to the threat of climate change, to resume efforts to limit temperature increase, and to increase the country capacity to deal with its impacts.
As a result, some researches were conducted a few years ago to discover a new technology to extract fuel and gasoline by mixing water and air extracts. This should lead to purifying air from its most dangerous elements, in addition to the provision of adequate amounts of fuel.
In reality, this technology did not result from this crisis; it is an old technology developed by the Germans in 1923, and is known as “synfuel” or “synthetic fuel”. In this process, a synthetic gasoline is extracted that is less polluted, with fewer impurities, and is not yellow in color as usual, because it does not contain sulfur. The odor of the extracted gasoline is similar to that of conventional benzene; however, it is purer, because it does not contain added or harmful elements.
Technically, this modern technology relies on extracting carbon dioxide (CO2) from air, and mixing it with extracted hydrogen (H2) from water. The mixture forms methanol, which is the main compound of gasoline and benzene; it is then stimulated by the Fischer–Tropsch process. This technique is named after its original German developers, Franz Fischer and Hans Tropsch, who used a fuel catalyst reactor of iron or cobalt after the distillation process to dispose water amounts and convert it into benzene in specialized reactors in 200–400°C temperature. Check the reaction in the figure below:
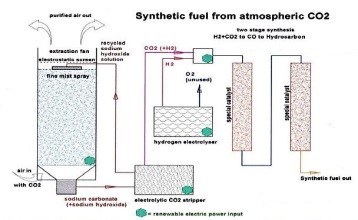 Source: https://img.newatlas.com/air-fuel-synthesis-gasoline-from-air-water-1.jpg?auto=format%2Ccompress&ch=Width%2CDPR&fit=max&q=60&w=616&s=7ac1d96f3945fa2918a4c85e689309f1
Source: https://img.newatlas.com/air-fuel-synthesis-gasoline-from-air-water-1.jpg?auto=format%2Ccompress&ch=Width%2CDPR&fit=max&q=60&w=616&s=7ac1d96f3945fa2918a4c85e689309f1
This technology enabled scientists in London, during 2013, to produce 50 liters of gasoline, and were actually used as a fuel of a motorcycle; however, it was somewhat expensive compared to fuel prices at the time.
You can also watch this video on the “development of synfuel product process from coal”. You can also check this recent study on "The Role of Synthetic Fuels for a Carbon Neutral Economy”, and its effect on the ecological balance, from this link.
Can this innovative technology solve the persistent fuel shortage, air pollution, and global warming issues; thus, achieving the Paris Climate Agreement goals? Or, will it be yet another incomplete experiment due to its high cost; thus, remaining practically unenforceable?
References
livescience.com
newatlas.com