| front |1 |2 |3 |4 |5 |6 |7 |8 |9 |10 |11 |12 |13 |14 |15 |16 |17 |18 |19 |20 |21 |22 |23 |24 |25 |26 |27 |28 |29 |30 |31 |32 |33 |34 |35 |36 |37 |38 |39 |40 |41 |42 |43 |44 |45 |46 |47 |48 |49 |50 |51 |review |
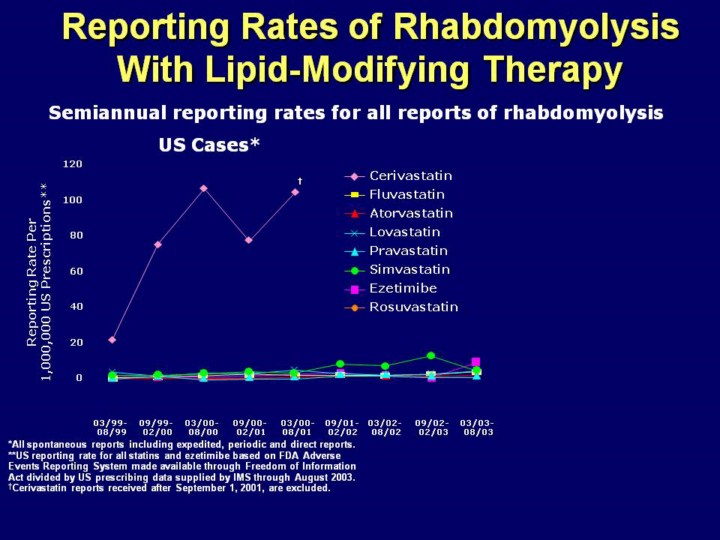 |
Key Point: Postmarketing surveillance clearly demonstrates that the rate of rhabdomyolysis for rosuvastatin is similar to that for other currently marketed statins. A comparison of postmarketing data for rosuvastatin, other statins, and ezetimibe has also been performed Rates of rhabdomyolysis for rosuvastatin were calculated in 6-month intervals for the past 12 months by dividing the worldwide number of spontaneous reports of rhabdomyolysis received by AstraZeneca by the worldwide number of prescriptions for rosuvastatin (using data from IMS). These rates represent the most recent and comprehensive information to date
Data for comparator statins and ezetimibe were obtained from the Federal
Drug Administration (FDA) Adverse Event Reporting System (AERS), available
to the public through the Freedom of Information Act (FOIA). Rosuvastatin is
not currently represented on this graph because the US data on FOI represent
the timeframe up to and including 08/2003 (rosuvastatin became available in
the
As shown here, the worldwide semiannual reporting rate for rosuvastatin is
in line with the This is particularly noteworthy because new drugs are expected to have higher reporting rates of adverse events in their first 2 to 3 years of launch As clearly depicted on the slide, the reporting rate of rhabdomyolysis for cerivastatin was much higher compared with the currently marketed lipid-lowering therapies The limitations of comparative spontaneous reporting rates are as follows The FDA AERS safety reports are spontaneous, not fully investigated, and do not imply a causal relationship Spontaneous reporting rates are likely to be lower than the actual incidence of adverse events due to underreporting Spontaneous reporting rates are susceptible to reporting bias, including an increase in reporting due to the “Weber effect”, increased publicity, and enhanced awareness of the potential side effects Spontaneous reporting rates of rhabdomyolysis for rosuvastatin, other statins and ezetimibe do not represent head-to-head comparative studies and must be interpreted with caution |