| front |1 |2 |3 |4 |5 |6 |7 |8 |9 |10 |11 |12 |13 |14 |15 |16 |17 |18 |19 |20 |21 |22 |23 |24 | 25|26 |27 |28 |29 |30 |31 |32 |33 |34 |35 |36 |37 |38 |39 |40 |41 |42 |43 |44 |45 |46 |47 |48 |49 |50 |51 |review |
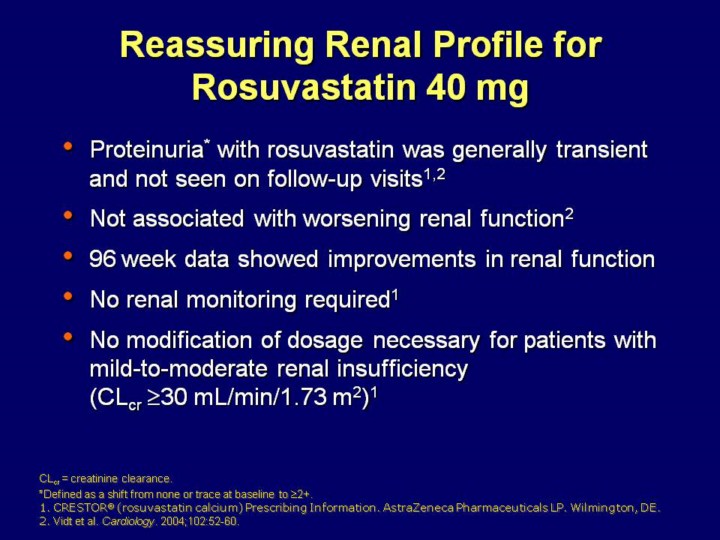 |
Key Point: Proteinuria appears to be a statin class effect. During controlled clinical studies with rosuvastatin, proteinuria was observed with all statins and placebo.
The proteinuria that occurred with rosuvastatin was generally transient and The proteinuria was not associated with worsening renal function1,2 No renal monitoring required
No modification of rosuvastatin’s dosage is necessary when treating patients
with
References
1.CRESTOR®
(rosuvastatin calcium) Prescribing Information. AstraZeneca
Pharmaceuticals LP. Wilmington, DE.
2. Vidt DG, Cressman MD, Harris S, Pears JS, Hutchinson HG. Rosuvastatin-induced arrest in progression of renal disease. Cardiology. 2004;102:52-60.
|