| front |1 |2 |3 |4 |5 |6 |7 |8 |9 |10 |11 |12 |13 |14 |15 |16 |17 |18 |19 |20 |21 |22 |23 |24 |25 |26 |27 |28 |29 |30 |31 |32 |33 |34 |35 |36 |37 |38 |39 |40 |41 |42 |43 |44 |45 |46 |47 |48 |49 |50 |51 |review |
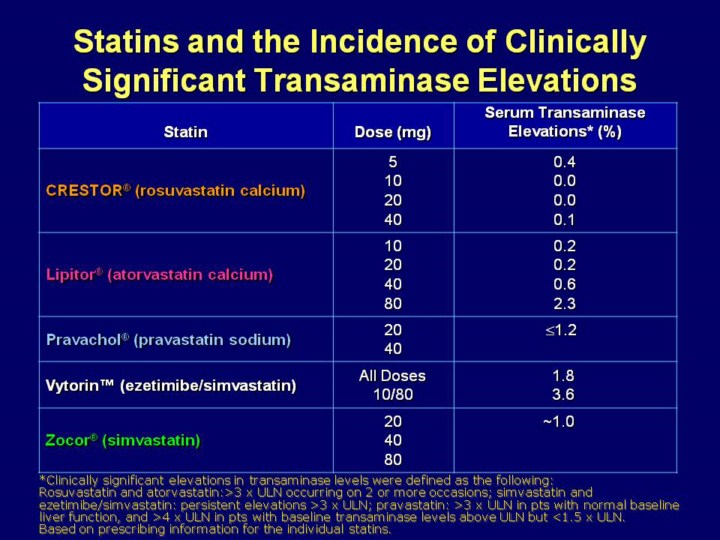 |
Key Point: The incidences of transaminase elevations with 4 of the most commonly used statins are low. Clinically significant transaminase elevations are infrequently observed with the most commonly used statins1-5. This slide shows the incidence of transaminase elevations reported in the labeling of rosuvastatin, atorvastatin, pravastatin, simvastatin, and ezetimibe/simvastatin, which ranged from 0% with rosuvastatin 10 mg and 20 mg to 3.6% with ezetimibe 10 mg/simvastatin 80 mg
Clinically significant elevations in transaminase levels were defined as
levels >3
´
ULN occurring on 2 or more occasions (rosuvastatin, atorvastatin),
persistent elevations The clinically significant transaminase elevations that occurred with these agents were usually transient and resolved or improved after treatment was interrupted or discontinued, as noted in the labeling information for rosuvastatin, atorvastatin, simvastatin, pravastatin, and ezetimibe/simvastatin1-3,5
References 1. CRESTOR® (rosuvastatin calcium) Prescribing Information. AstraZeneca Pharmaceuticals LP. Wilmington, DE. 2. Lipitor® (atorvastatin calcium) Prescribing Information. Pfizer Inc. New York, NY. 3. Pravachol® (pravastatin sodium) Prescribing Information. Bristol-Myers Squibb Company. Princeton, NJ 4. VytorinÔ (ezetimibe/simvastatin) Prescribing Information. Merck & Co., Inc. Whitehouse Station, NJ. 5. Zocor® (simvastatin) Prescribing Information. Merck & Co., Inc. Whitehouse Station, NJ. |